T1D Guide
T1D Strong News
Personal Stories
Resources
T1D Misdiagnosis
T1D Early Detection
Research/Clinical Trials
Are Over-the-Counter CGMs Good for All?
The American Diabetes Association (ADA) recently held its 85th Scientific Sessions at McCormick Place Convention Center in Chicago, IL. At this event, leading voices in diabetes came together to explore recent innovations, share updates on technology and news, and engage in networking, panel discussions, and debates. The ADA covered topics like obesity, diabetes complications, clinical trials, metabolism, GLP-1s, and misdiagnosis theories. However, one topic stood out in particular: over-the-counter (OTC) continuous glucose monitors (CGMs)—a hot debate within the diabetes community.
.jpg)
Spotlighting a Hot Topic in Diabetes
Better known as glucose biosensors, these devices lack the robust features and functionality of traditional continuous glucose monitors (CGMs) that are designed to support people with diabetes across a broader range of blood sugar levels. Glucose biosensors are not intended to support people using insulin or those who experience hypoglycemia (low blood sugar).
On this topic, one notable debate at the 85th Scientific Sessions focused on: “Over-the-Counter Continuous Glucose Monitoring—Data for All or a Disaster Waiting to Happen?”
When it comes to the general public using OTC CGMs, opinions within the diabetes community are mixed, as evident during the scientific sessions, especially during this discussion.
Many individuals with diabetes have taken to social media to express their concerns about glucose biosensors. Some diabetes advocates and influencers compare the use of these biosensors in people without diabetes—or those who do not experience hypoglycemia—to wearing a pacemaker without having heart issues.
Others worry that glucose biosensors could lead to disordered eating patterns in individuals who may not receive proper nutritional education or fully understand how their blood glucose levels should function, among other potential risks.
On the other hand, some individuals in the diabetes community support the availability of over-the-counter (OTC) CGMs, arguing that they could reduce the costs associated with purchasing CGMs through pharmacies or durable medical equipment (DME) suppliers in the long run, among other advantages.
During this lively debate, two medical professionals represented each side, highlighting key points worth considering beyond those mentioned above.
Is CGM Data for All Really Good for All?
Rayhan Lal, who has been living with type 1 diabetes for over 30 years, served as the debate moderator. Rayhan is an assistant professor of Medicine/Endocrinology at Stanford University.
Diana Isaacs, PharmD, BCPS, BCACP, BC-ADM, CDCES, presented the “Data for All” section of the panel, which highlighted the benefits of glucose biosensors. Diana serves as the Director of Education and Training in Diabetes Technology at the Cleveland Clinic. She is a consultant for several companies, including Lilly, Novo Nordisk, Sanofi, Mannkind, Insulet, Tandem, Medtronic, Dexcom, Abbott, Corcept, Cequr, and Sequel.
Diana encouraged attendees to use the hashtag “#data4all” to engage in discussions about the presentation on social media while following live updates. For those catching up later, social media posts can now be viewed as an archive, allowing the conversation to continue.

Blood Glucose Monitors Weren’t Always Available Over-the-Counter Either
“Believe it or not, there was a time when blood glucose monitors (BGMs)…those were only available in doctor’s offices,” Diana said. “When they became over-the-counter, in the 1980s it was like Woah!’ And we laugh at that. They shouldn’t only be hiding them in doctor offices, right?”
“When CGM first became available, it was only available to the super privileged people who had the money able to afford them and the healthcare providers willing to prescribe them,” Diana said. “We’ve seen over time how accessibility improved.”
Diana explained that there was a time when individuals using CGMs had to take multiple insulin injections each day and demonstrate the number of fingersticks they performed to qualify for a CGM. Over time, policies evolved to allow all insulin users to access CGMs more easily. Now, in many areas, CGMs are widely available through insurance, regardless of the diabetes medications a person is using.
2025 marks a new era for CGMs. Over-the-counter CGMs can be ordered online, don’t require a doctor’s prescription, and work with mobile apps that feature lifestyle education and glucose goal-setting. Only adults over the age of 18 can use these devices. Again, they are not intended for use in insulin-dependent individuals or individuals who experience hypoglycemic episodes.
Glucose biosensors do not supply alerts for hypoglycemia.
A New Era for CGMs: Dexcom Stelo and Abbott Lingo Lead the Way
In the U.S., popular OTC CGM options include Dexcom Stelo and Abbott Lingo. Although not mentioned in Diana’s presentation, several other brands, such as Nutrisense or Signos, offer their own versions. If you’re active on social media and have expressed interest in CGMs or glucose biosensors before, you might have seen ads for these products.
Dexcom Stelo is designed for adults who are not on insulin and have type 2 diabetes, prediabetes, or are interested in tracking their glucose levels, Diana explained. While Abbott Lingo is designed for adults who are not on insulin and are interested in understanding how to improve their metabolic health, she furthered.
Sharing abilities, real-time alerts, special features, glucose reading frequencies, in-app education, age indications, and costs–vary between these products.
“The key thing is they don’t have a ton of alerts to bother people,” Diana said.
Stelo provides "Spike Alerts" to notify users when their glucose levels are rising at a rate that the app identifies as a "Spike." In contrast, Lingo features a program called "Lingo Count," which assigns points to users for increases in their blood glucose levels throughout the day.
The goal is to accumulate as few points as possible, serving as a coaching tool for users.
Diana also mentioned that Abbott Rio received FDA clearance last summer, but it is not yet available for purchase. This sensor will have a range of 40 to 400 mg/dL, which exceeds the glucose biosensor ranges of Abbott Lingo and Dexcom Stelo.
What Do the ADA’s Standards of Care Say?
Diana argued, “Who shouldn’t be using CGM?”
The ADA’s Standards of Care urge clinicians to “consider the use of CGMs in adults with T2D not on insulin therapy, to achieve and maintain individualized glucose goals.”
.jpg)
Diana discussed the numerous challenges that glucose biosensors address, including the barriers to access caused by high costs or lack of insurance coverage, as well as the infrequency of doctor check-ins that may otherwise necessitate a prescription.
Since glucose biosensors are more accessible, their availability may be more likely to inspire lifestyle changes, Diana concluded.
CGM Use Cases for Different Groups of People
Diana shared use cases for over-the-counter CGMs among key groups. She highlighted:
Prediabetes
In the U.S. and the world, there is a huge problem—a prevalence in prediabetes. Many people are unaware they’re even living with it. And while OTC CGMs aren’t diagnostic, they can help provide the data needed to obtain a diagnosis. CGMs can be a valuable tool for promoting change in food habits, physical activity, sleep, and stress management.
“There are so many benefits to early intervention,” Diana said.
Obesity
Meanwhile, Diana shared that OTC CGMs can share time in range (TIR) data with people who have obesity and are not currently living with diabetes, to facilitate lifestyle changes that support better health outcomes.
Find out how to improve your Time in Range with these 9 Tips.
Gestational Diabetes
“The current standard of practice has been fingersticks,” Diana shared. In a randomized control trial in Switzerland involving women with gestational diabetes, there was no difference in the primary outcomes between blood glucose monitoring (BGM) and continuous glucose monitoring. However, participants reported a higher quality of life when using a CGM.
Diabetes without a Prescription or Insurance Coverage
Insurance coverage may restrict the number of CGM sensors that an individual can receive each month. Healthcare providers (HCPs) often advise people with diabetes to contact the company directly to request additional sensors, but this approach doesn't always yield results. Although it's not a perfect solution, over-the-counter CGMs can provide relief when insurance does not support a person with diabetes in obtaining the necessary number of sensors in a timely manner.
Athletes
Diana shared that emerging data suggests the use of OTC CGMs in athletes can enhance their athletic performance.

Healthy People
You might consider this group as representing the "average Joe." The general population has an interest in health data. With the ability to pair products like the Oura Ring, individuals can become more attuned to their bodies.
Pre-stage 3–Type 1 Diabetes
Finally, in monitoring early-stage T1D, OTC CGMs can help prevent diagnosis in diabetic ketoacidosis (DKA).
In conclusion, Diana shared that:
- “OTC CGMs are effective for multiple groups of people.”
- “(OTC CGMs) can facilitate follow-up testing for diagnosis or disease progression, empower people to make positive lifestyle changes, and improve A1C and time in range.”
- “People deserve to have access to their data.”
The Case Against CGMs for All
David Ahn, MD, Chief of Diabetes Services at the Mary & Dick Allen Diabetes Center at Hoag Memorial Hospital Presbyterian, presented arguments against using CGMs for everyone following Diana’s passionate presentation. “#CGMsforDiabetes?” he posed.
David is an advisor for Lilly, Ascensia, Honey Health, and Mannkind. He is also a speaker for Abbott, Ascensia, Insulet, Lilly, MannKind, Novo, Xeris, and Sequel MedTech.
“It’s really important for us to think about the history of CGM,” David said. “CGM was invented for people with diabetes. And over time—and look, I’m equally excited as Diana—we’ve been able to expand access to CGM. And now we’re starting to expand even more so in the wellness and metabolism spaces.”
“Still, I’m warning you, there’s some dragons to watch out for when it comes to over-the-counter CGMs,” David said.
Is There Enough Evidence or Support for OTC CGM Use in Gen Pop?
David argued that there is less evidence to support OTC CGMs in people without diabetes or among those who don’t experience hypoglycemic episodes.
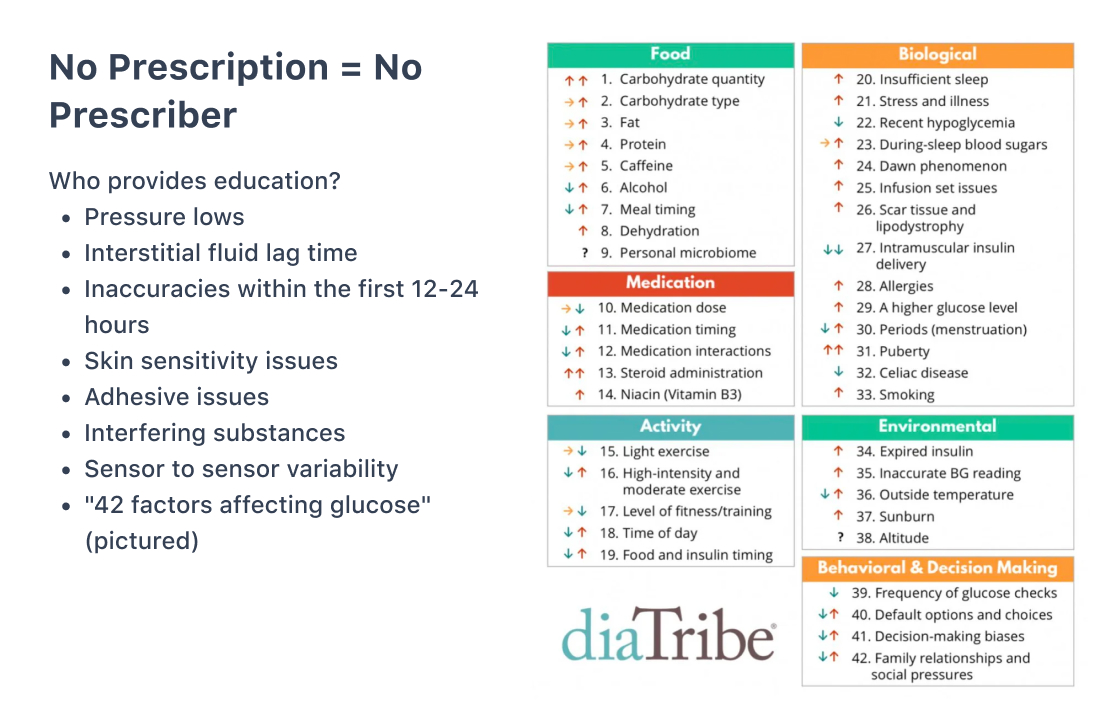
“While it’s exciting that the OTC CGMs don’t require a prescription, that also means you don’t have a prescriber,” David shared. “That means you don’t have education or guidance along with those things. So the question becomes ‘Who provides that education or guidance?’”
“CGMs are not without their flaws,” David said.
David shared the following, which are not always accounted for or under-emphasized in in-app OTC CGM training:
- Pressure lows
- Interstitial fluid lag time
- Inaccuracies in the first 12-24 hours
- Skin sensitivity issues
- Adhesive issues
- Interfering substances
- Sensor to sensor variability
“There are 42 factors affecting glucose,” David said. “How can we expect people who are purchasing these on their own to interpret these numbers? We know these sensors have flaws, even within the intended communities. My concern is that this (general) population won’t appreciate the nuances associated with CGM.”
Marketing to Metabolic and Wellness Communities
Marketing campaigns tease that you can unlock the secrets of your body, but “unfortunately, it’s often not that simple,” David concluded.
In a series of studies, David shared that participants without diabetes ate various meals while wearing different sensors. The study showed there was variability in glycemic response between the same person eating the same meal, contradicting the marketing campaigns David mentioned.
“It very much calls into question how well these sensors are actually going to be able to differentiate between blood sugar responses to different meals,” David said.
Leading glucose biosensors share information about normal glucose spikes, but what’s normal for individuals may vary based on stress levels, eating a high-carb meal, and other factors.
“If you’re trying to vilify spikes, then the question is ‘what is the ultimate goal for our patients?’” David pondered. “Is our ultimate goal not to have any spikes at all and to have a flat line? Surely that’s not the case, because essentially you’d be encouraging people not to eat or follow an extremely low-carb diet…which isn’t right for every person.”
David concluded that, for these reasons, glucose spikes can be confusing for people without diabetes.
What Normoglycemia Should Look Like
In people with non-diabetes, David shares that there is an undefined “healthy” time in range, though various studies have attempted to shed light on what’s normal for people without diabetes. What’s healthy for one person might not be right for another.
David shared that Stelo, for example, may reference a study where 96% of people fell in a target range of 70 to 140 mg/dL. Another paper found the same. Shah’s study required calibrations; however, DuBose’s did not.
“When it comes to prediabetes, of course, we know the fasting blood sugar should be between 100 and 125 mg/dL,” David stated. “These systems are explicit in saying they’re not intended for diagnosis tools, but I think people will naturally tend to do that.”
To summarize, David said that:
- “OTC CGMs encourage the use of biosensors in evidence-free zones such as wellness that overpromise and under-deliver.”
- “‘Glucose spikes’ and misinterpreting data can lead to confusion, anxiety, and worse, disordered eating.”
- “OTC CGMs are not designed for accuracy in the narrower band of euglycemia (70 to 180 mg/dL).”
For example, misunderstandings may lead to misinterpretations of food and the body’s response in people without diabetes. David later featured this example in his rebuttal:
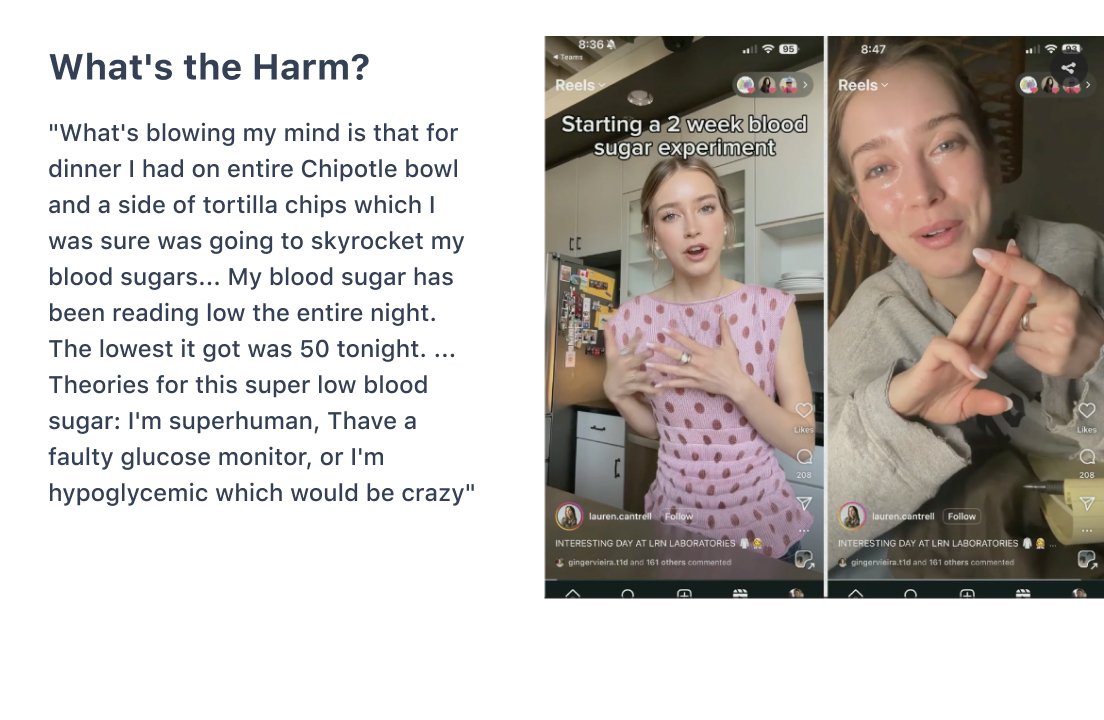
Watch Debates Like This On-Demand
These powerful arguments touch the surface of the debate between CGM access for all versus CGM access only for people with diabetes or those who experience hypoglycemia.
Conversations like this help propel the future of diabetes care and general healthcare. If you want to replay exhilarating debates like this, there’s still a chance to catch up on demand through the ADA portal.
Whether you were on-site or attending virtually, catching everything in real-time was nearly impossible, but the ADA on-demand portal makes it easier to catch up. You can still register to view the sessions virtually here.
When you sign up, you can access the content through August 25, 2025, and earn up to 29.5 CME/CE credits. Enjoy reliving or viewing for the first time the exciting panel discussions, educational presentations, and abstracts! You’re sure to learn a lot (and have some juicy tidbits or talking points to share at your next networking event).
Is There a Winner in this Debate?
Data suggests that continuous glucose monitors can benefit everyone, but there are also arguments against this. Even experts can’t come to a consensus in 2025! Who will prevail in this debate? Only time will reveal the outcome as further monitoring, user insights, research, and reviews unfold.
“All this being said, there’s definitely work to be done in this space,” moderator Rayhan Lal finished.








.webp)
.jpg)
.jpeg)
.jpg)
.webp)
.jpg)
.jpg)
.jpg)


.jpg)


.jpg)



.jpg)
.jpg)
.jpg)

.jpg)

.jpg)














.jpg)


.jpg)







.webp)












.webp)





















.webp)








.jpg)




.jpg)
















.webp)